
Host Response
A major obstacle in clinical oncology is that tumors eventually acquire resistance to their therapy. The mechanisms by which resistance arises are usually associated with changes that occur in the tumor cells in response to therapy. We pioneered the hypothesis that host factors secreted in response to therapy comprise pro-tumorigenic effects. Such factors can promote tumor cell repopulation, angiogenesis, and metastases, and therefore may contribute to tumor resistance or recurrence following therapy. Several previous studies, from our laboratory, demonstrated that following chemotherapy, radiation, surgery and even targeted drugs, the drug generates host responses which in turn contribute to resistance and outgrowth of tumors. Different aspects of this phenomenon is being studied in the lab, with the goal to identify new targeted therapies to overcome drug resistance. These targets are based on the host reaction to the therapy and not on tumor cell killing effect.
Thus, our research serves as a starting point for generating new targeted therapies for cancer which are based on the inhibition of host factors promoting tumor resistance to therapy.
Related Projects
Host response to immunotherapy induces blood-brain-barrier disruption
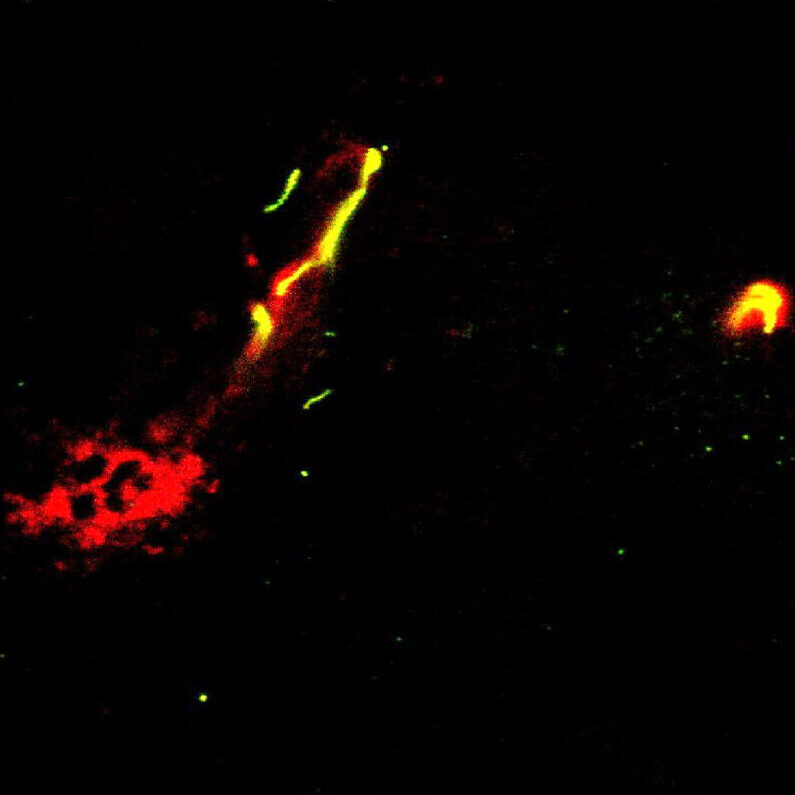
Host response to chemotherapy supports pulmonary metastasis in part due to the formation of pre-metastatic niche
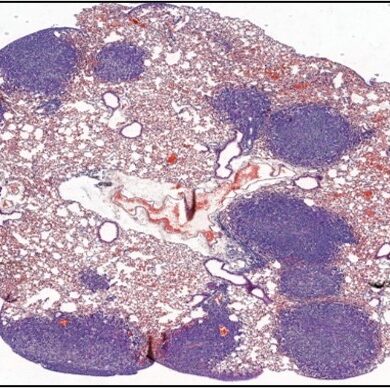
Host response to immunotherapy contributes to pulmonary metastasis
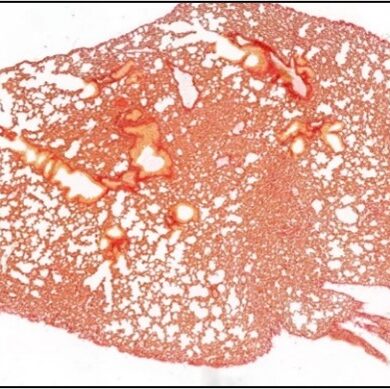
ECM Remodeling
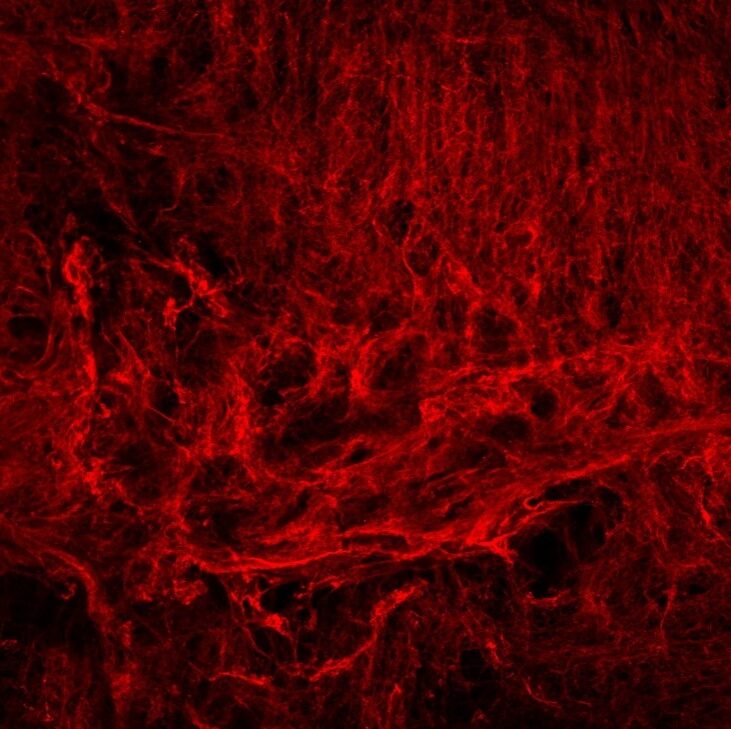
We study how anti-cancer drugs can induce host-mediated mechanisms that contribute to remodeling of the extracellular matrix (ECM) either in the lungs or at the tumor site. These effects, as we previously shown, facilitate cancer cell seeding and metastasis. For example, we have recently shown that paclitaxel (PTX) chemotherapy enhances rapid ECM remodeling and mechano-structural changes in the lungs of mice. We also demonstrated that these effects are mediated by CD8+ T cells expressing lysyl oxidase (LOX). These studies highlight the role of immune cells in regulating ECM and metastasis following anti-cancer drug therapy including chemotherapy and immunotherapy. We also suggest that inhibiting therapy-induced ECM remodeling can present a therapeutic strategy to overcome metastasis.
Related Projects
Bone marrow cells contributing to ECM remodeling
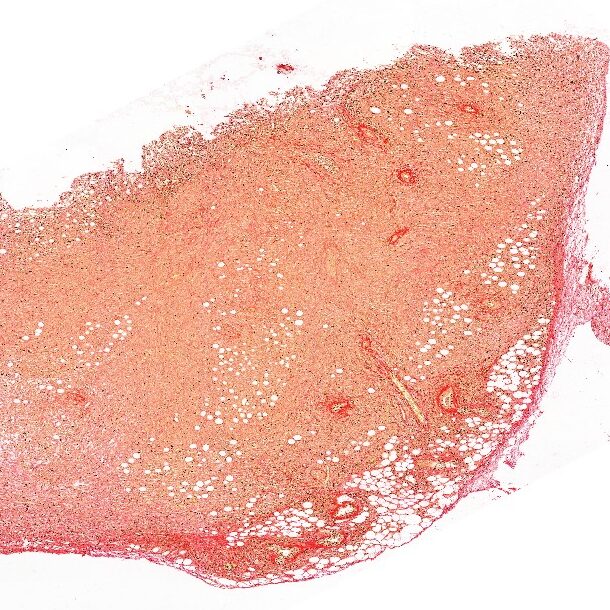
ECM remodeling in response to anti-cancer therapy

Cancer Immunotherapy
Cancer immunotherapy has undergone a quantum leap in recent years as a result of the elucidation of the mechanisms underlying immune escape of cancer cells. While using immune check point inhibitors (ICIs) therapy show tremendous antitumor immunological effects, often manifested with full recovery, still only approximately 20%-30% of patients respond or benefit from this therapy. In addition, resistance and/or hyper-progression of the cancer sometimes could be ensued after therapy, which could result in tumor expansion and growth. Here we study the mechanisms leading to enhanced aggressiveness of tumors after immunotherapy and the reasons for metastasis. In addition, we also search for biomarkers that can predict outcome therefore supporting clinical decision making using immunotherapy. Our approach may result in identifying ways to increase the percentage of patients that can benefit from ICI therapy and help those that display resistance.
Related Projects
Identifying biomarkers for immunotherapy response
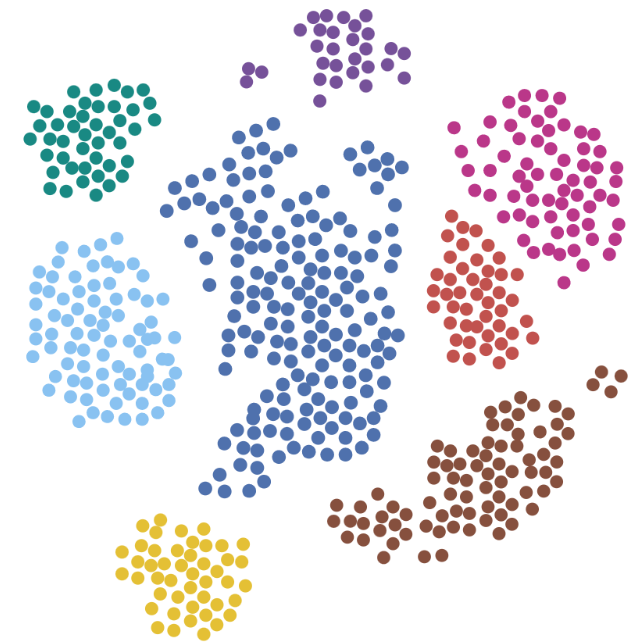
Improving immunotherapy outcome in resistant tumors
Exploring immunotherapy drug combinations and their impact on cancer
Drug Discovery
Multiple myeloma (MM) is a progressive malignancy of plasma cells with poor survival rates. Treatment with proteasome inhibitors, such as bortezomib, is considered a breakthrough in MM care. However, while bortezomib significantly improves survival, some patients fail to respond for reasons such as de-novo resistance, low pharmacokinetics, or off-target adverse effects. Our study focuses on the development of new generation of bortezomib to increase therapeutic outcome. We develop bone-marrow targeted liposomes encapsulating bortezomib and explore their therapeutic activity in MM for human testing. We study the efficacy of this drug compound on MM bearing mice, and developing it towards new therapeutic strategy of MM to be tested in humans.

Related Projects
The development of targeted therapy for multiple myeloma

The development of antibody-drug conjugate to improve immunotherapy outcome


